Introduction: Innovating Under Regulatory Scrutiny
Medical electronics design demands a razor-sharp balance between cutting-edge technology and compliance with global standards like FDA 21 CFR Part 820 and EU MDR. McKinsey reports that 40% of medical device delays stem from design-stage compliance gaps—a risk that derails both timelines and budgets.
This article dissects five critical challenges in medical electronics development, illustrated by OPD-Design’s success in securing FDA 510(k) certification for an Infrared Hair Growth Helmet. Download our free Medical Compliance Toolkit to fast-track your product launch.

III. Case Study: FDA 510(k) Certification for Infrared Hair Growth Helmet
Client Profile
- A U.S. biotech startup developing a home-use Low-Level Light Therapy (LLLT) device targeting androgenetic alopecia.
- Goal: Achieve FDA Class II clearance for consumer markets.
Key Challenges
- Technical Compliance:
- FDA required proof of biological safety (650-850nm infrared wavelengths) and clinical efficacy via 6-month human trials.
- EU MDR mandated compliance with IEC 60601-1 electrical safety standards.
- Manufacturing Risks:
- Optical module yield <50%, tripling per-unit costs.
OPD-Design’s Breakthrough Strategy
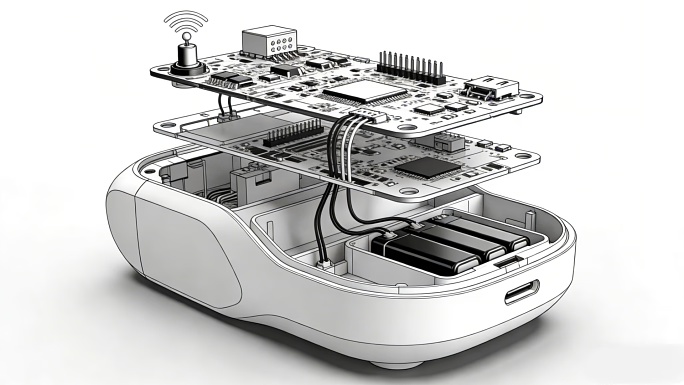
1. Hardware Redesign & Validation
- Optical System Optimization:
- Redesigned multi-channel LED arrays reduced light intensity variance from 22% to <5%.
- Integrated temperature sensors ensured scalp contact surfaces stayed below 41°C (per IEC 60601-2-57).
- EMC Mitigation:
- Deployed common-mode chokes on PCB power layers, cutting radiated emissions below FCC Class B limits.
2. Accelerated Clinical Validation
- Alternative Data Strategy:
- Leveraged existing clinical data from predicate devices (e.g., Capillus®) combined with in-vitro hair follicle assays to reduce human trial requirements.
- Streamlined Testing:
- Partnered with FDA-accredited labs to slash biocompatibility testing from 12 to 6 weeks.
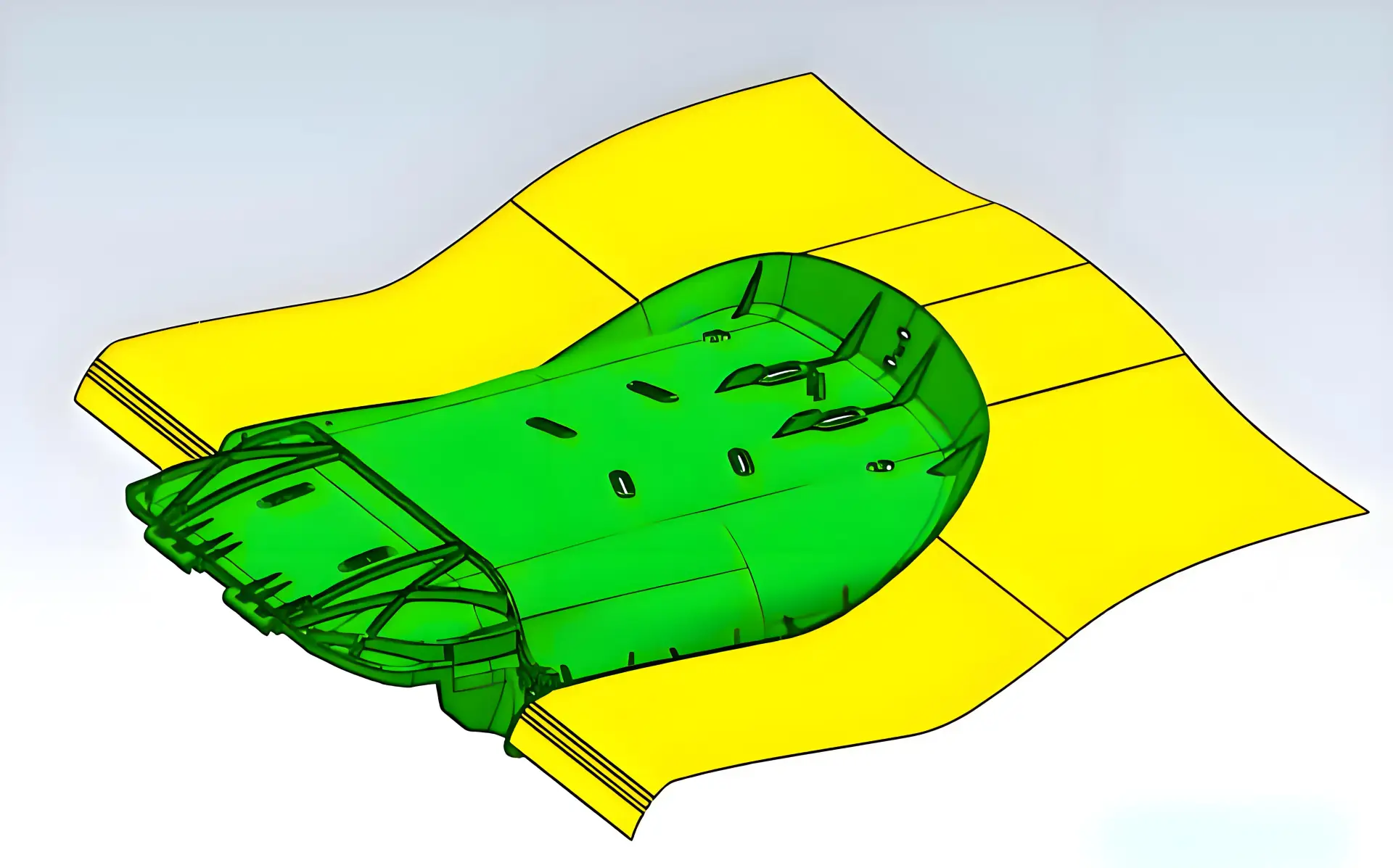
3. Design for Manufacturing (DFM)
- AI-Powered Calibration:
- Developed computer vision systems to auto-adjust LED drivers on production lines, boosting yield to 92%.
- Modular Architecture:
- Decoupled optical cores from housings, enabling hybrid molding/3D printing and cutting tooling costs by 47%.
Results
- FDA 510(k) clearance in 5 months (industry average: 10-14 months).
- Production costs reduced by 35%, with 120,000 units shipped in Year 1.
- Achieved EU MDR certification for Germany, France, and Benelux markets.